Review of Cannabis Pharmacology, Uses, Adverse Drug Events, and Drug Interactions
In a recent review led by Joshua Brown, PharmD, PhD, researchers at the University of Florida describe the current environment for natural and synthetic cannabinoid use.
Published in The Journal of Clinical Pharmacology, the review provides context for the chemistry and effects (or pharmacology) of cannabinoids related to health including a summary of approved uses and action for adverse drug events.
The cannabis plant contains over 100 known natural cannabinoids. The two most common cannabinoids are cannabidiol (CBD) and trans-Δ9-tetrahydrocannabinol (THC). Cannabinoids interact with the body’s endocannabinoid system (ECS) which balances the body’s sleep cycle, hormone levels, appetite level and more.
For the review, natural cannabinoids included cannabinoids directly from the plant and any cannabinoid directly synthesized to be identical to natural cannabinoids. Synthetic cannabinoids are purely synthetic in nature and may be found in “Spice,” “K2,” fake weed, and legal high products.
Data indicates the synthetic cannabinoids produce effects like THC but are generally more potent and lead to more severe reactions. Unlike natural cannabinoids, synthetic cannabinoids have not been thoroughly studied. There are currently four prescriptions approved worldwide containing natural cannabinoids and one containing a synthetic cannabinoid.
These prescriptions are used for variety of conditions including cancer-related pain and nausea and vomiting associated with chemotherapy, multiple sclerosis pain, severe seizers, and anorexia associated with HIV/AIDS.
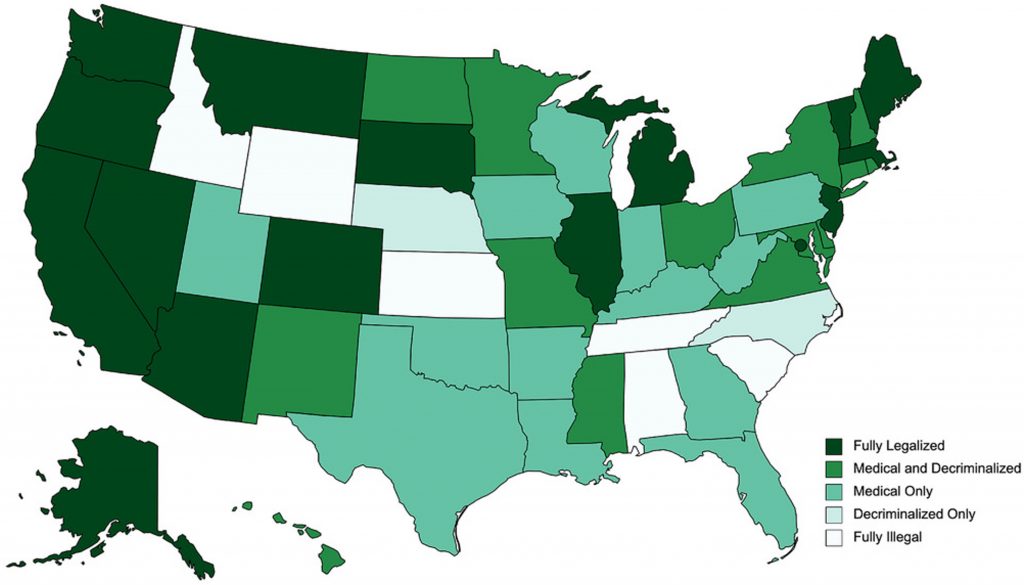
The availability and access to the prescriptions and non-prescription cannabis products is varied and inconsistent across the country and world. CBD possession and sales are generally legal through the 20 “Farm Bill” which legalized low-THC (<0.3% THC) hemp cultivation. In the United States, all THC-based products remain a Schedule 1 drug at the federal level. As of March 2021, 14 states have medical programs.
Despite available research and general knowledge, cannabis and cannabinoids are pharmacologically complex and pose risks for adverse drug events and drug-drug interactions. Current research is mixed on the potential benefits and risk related to cannabis therapies. The conditions with stronger evidence supporting the use of cannabis as treatment or therapy is limited to seizure disorders such as epilepsy, chronic pain, and multiple sclerosis.
While evidence from research lags, potential for adverse drug events and drug-drug interactions remains evident. A recent meta-analysis of cannabis clinical trials found that the risk of adverse drug events was 3-fold higher for cannabis therapies compared to placebo.

Special consideration about adverse drug events for medical marijuana patients are key because many patients have risk factors including other medications for health conditions, severe or life-altering conditions, and more. Additional special populations including people who are or become pregnant, pediatrics and adolescents, and older adults and geriatric patients.
In conclusion, further evidence and education are essential to understanding the potential benefits and to preventing the harms of cannabis and cannabinoid use.
Read the full article in The Journal of Clinical Pharmacology.
