MEMORY Quicklinks
Data Sharing Process
MEMORY has been conceived to establish the infrastructure to generate real-world evidence on the use and outcomes of medical marijuana (MMJ). For this purpose, the Consortium is linking the Office of Medical Marijuana Use (OMMU) Medical Marijuana Use Registry (MMUR), with Florida vital statistics data (e.g., birth and death certificates), Florida Medicare and Medicaid claims databases, and other relevant data commonly used for health outcomes and policy research to create a robust research-ready repository. The Consortium makes a de-identified version of the repository available to Consortium researchers, thus providing state-wide infrastructure for real-world clinical outcomes research.
This page includes information about the process to request data, a summary of proposed, ongoing, and completed projects, and a list of Scientific Oversight Committee members. MEMORY has a central administrative system with procedures to review proposals, avoid duplication of work, provide appropriate opportunities for authorship, and monitor the output (papers, abstracts, etc.).
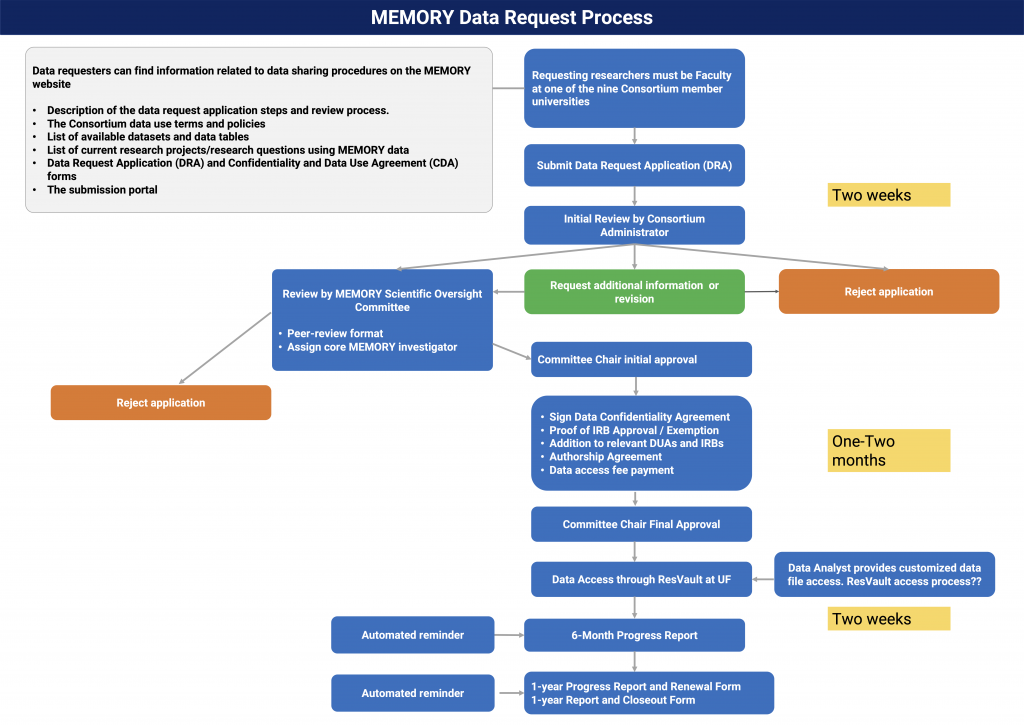
MEMORY Data Request Application Review and Approval Process
- Principal Investigators (PI) complete a Data Request Application to provide information for using the MEMORY data and provide their contact information, a tentative manuscript title, a clear research question they aim to address, dataset and variables requested and a list of authorized data users etc.
- A Consortium Application Administrator will review to verify the fit of the applications and ensure no overlap between ongoing manuscripts.
- The Administrator will then move the application for review by the Scientific Oversight Committee, contact the PI for additional information, or reject the submission. The Scientific Oversight Committee currently consists of 2 members from the University of Florida (UF) and in future will have members from outside UF who must have appropriate expertise/experience with use of survey and/or electronic healthcare data and be willing to serve on the committee. Each application will be forwarded to these reviewers, including those from outside UF.
The committee will review the submitted applications as they are received based on the following:
- Rigor of the study
- Appropriateness of the data for the suggested purpose and
- Potential overlap with other approved or planned studies (coming soon)
- To receive data, IRB approval is needed, and the investigator(s) will need to sign a confidentiality disclosure agreement and be added to relevant MEMORY IRBs and DUAs, sign an authorship agreement and pay the data access fee and analyst time for data retrieval.
- The Consortium Administrator tracks project progress over time, requesting updates every 6 months. The PI is responsible for ensuring that submitted abstracts and/or manuscripts include appropriate acknowledgements and disclosures. They are expected to share any new variables that they create from MEMORY data, along with the coding used to create the new variable.
- Prior to the expiration of the data access term, the PI submits a DRA extension request or DRA close-out request.