MEMORY Quicklinks
About MEMORY | Data Linkages and Data Architecture | Data Sharing Process | Planned, Ongoing, and Completed Research Projects
About MEMORY
The growing use of medical marijuana (MMJ) in Florida offers a unique opportunity for real-world evaluations of MMJ clinical outcomes that can help overcome the slow availability of randomized clinical trials and provide timely evidence on MMJ risks and benefits. The University of Florida, leads the Consortium of Medical Marijuana Clinical Outcomes Research (Consortium) established by Section 1004.4351, Florida Statutes. Nine additional universities in Florida are members of the Consortium to date. The Medical Marijuana Clinical Outcomes Research Data Repository (MEMORY) is a data bank developed by the Consortium to facilitate safety and effectiveness studies pertinent to MMJ use for certain qualifying medical conditions (knowthefactsmmj.com/patients). MEMORY allows for the longitudinal ascertainment of health outcomes, considering MMJ dose and routes, concomitant medication use, and comorbid health conditions for a population of Florida residents.
The MEMORY database, built progressively over several years, is evolving into a rich source of clinical outcomes research data (Figure 1). Early efforts in building this repository have focused on integrating important data sources, including MMJ certification and dispensing records from the Florida Department of Health’s (DoH) Office of Medical Marijuana Use (OMMU), Florida State Medicaid claims data, and vital statistics records.
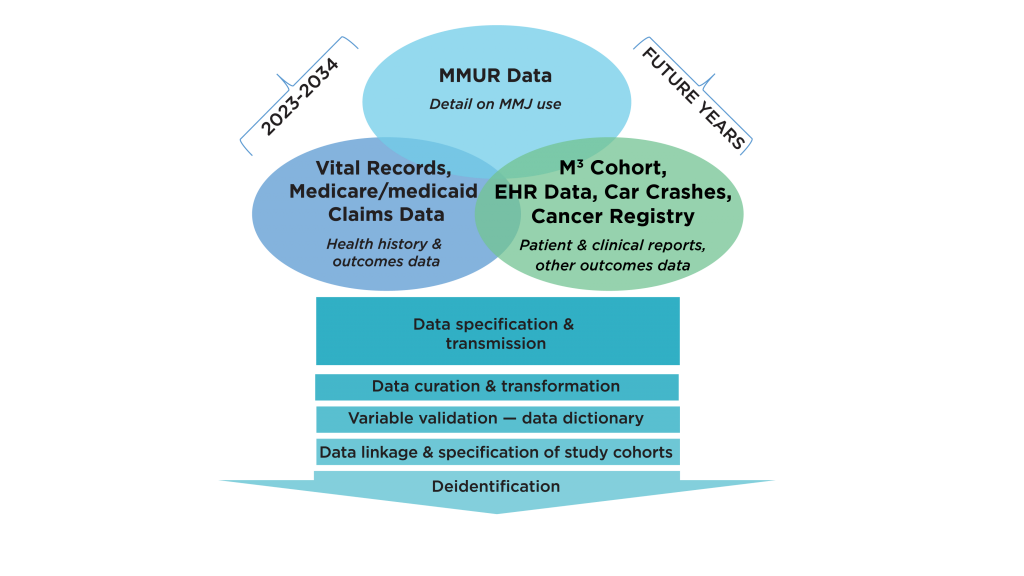
Figure 1. MMJ Clinical Outcomes Research Repository: MEMORY
(available to all Consortium members)
MEMORY can help overcome the lack of randomized clinical trials and greatly accelerate the availability of evidence on clinical outcomes. It has been conceived to establish the infrastructure for real-world MMJ clinical outcomes evaluations similar to those employed by the FDA to evaluate and monitor the risk-benefit of approved prescription medications. To ensure comprehensive longitudinal follow-up and capture of relevant health outcomes, the Consortium aims to link the OMMU Medical Marijuana Use Registry (MMUR) with other clinical databases commonly used for outcomes research. The Consortium plans to make a de-identified version of the repository accessible to Consortium researchers, thus providing state-wide infrastructure for real-world clinical outcomes research.
In the past years, the Consortium has obtained IRB approval and established Data User Agreements with the Agency for Healthcare Administration (AHCA), OMMU, the Centers for Medicaid and Medicare Services (CMS), and the Department of Health Vital Statistics Office for databases that will be linked to the MMUR. The Data User Agreement with the Department of Health OMMU to access the MMUR was signed early 2023.
The MEMORY data science team completed development of the data architecture, and has received the data for years 2016 through 2023 to facilitate the development of MEMORY.
The following figure shows the total number of unique individuals in different databases used in MEMORY development, and also the number of unique individuals linked with MMUR data.
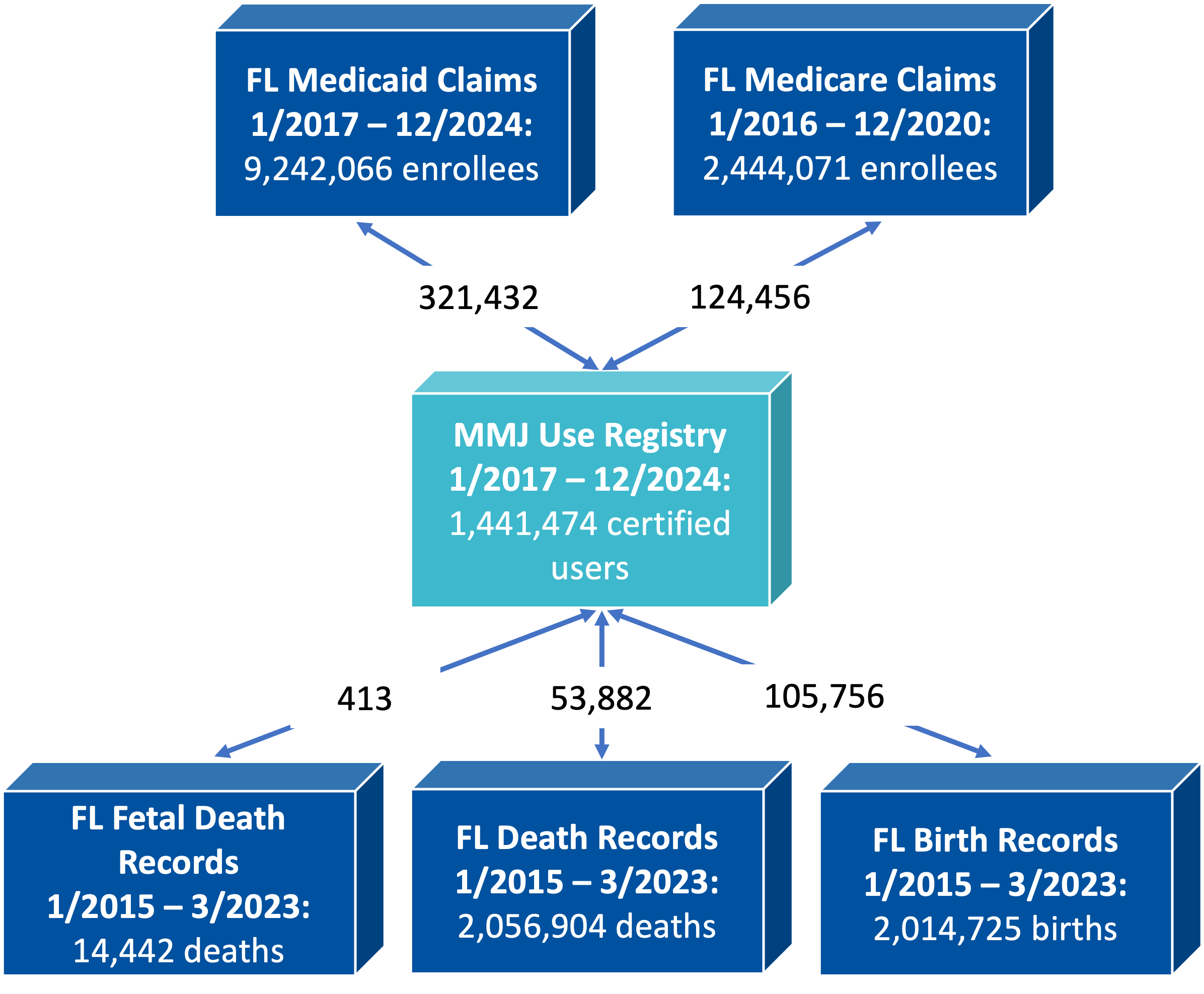
Figure 2. Total numbers by data source and total number of linked individuals.
MEMORY will support:
- controlled studies on MMJ effectiveness and safety
- active surveillance to capture emerging safety issues involving individual products or generalized effects among MMJ users
- MMJ utilization studies on MMJ access and utilization pattern across Floridians
Examples of research questions that could be addressed by MEMORY include:
- Safety of MMJ a in a variety of sub-populations defined by clinical indication or specific high-risk status considering a variety of adverse events and various doses, routes, concomitant medications and other factors such as comorbidities that may alter effects.
- Effectiveness and comparative effectiveness of MMJ for a variety of indications considering dosing and routes and various patient characteristics that may affect outcomes.
- Effects of MMJ certification standards on access, utilization pattern and outcomes of MMJ.
- Active surveillance of health issues related to route of administration of MMJ products.
Request Data Access
To request access to MEMORY data, please reach out to mmj.outcomes@cop.ufl.edu to complete a Data Request Application (DRA) and a Confidentiality and Data Usage Agreement (CDA).
Please visit Data Sharing Process for additional details.


