The Consortium for Medical Marijuana Clinical Outcomes Research works with the Florida Office of Medical Marijuana Use (OMMU) on the extraction specification and secure transmission of medical marijuana user registry (MMUR) data to the University of Florida (UF), which is made available to the Consortium under Sections 1004.4351 and 381.987, Florida Statutes on a quarterly basis. Once received, data are transformed into specific research variables that allow detailed descriptions of MMJ exposure, such as product types, dose, route, timing of treatment initiation and duration, and other information available in the registry.
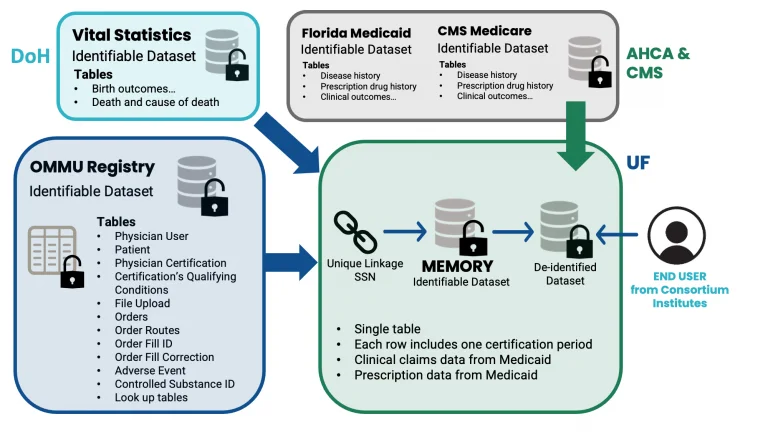
Figure 1. Data flow for MEMORY
Once OMMU data is received by the MEMORY development group within the Consortium, individual patient records can be linked to medical claims, and vital statistics data based on individual patient identifiers (Figure 1). While the honest broker has access to identifiable information for data linkage purposes, all these variables are encrypted (i.e., 18 HIPPA identifiers will be removed) for researchers. Authorized users for MEMORY will get access to a de-identified dataset of MEMORY, which will be housed at UF.
The MEMORY data architecture (Figure 2) represents a comprehensive network of linked tables available in MMUR data, including patients’ demographic details, qualifying medical conditions, medication orders, physicians’ certifications, controlled substance records, and any reported adverse events. This schematic is designed to efficiently organize complex datasets that facilitate a robust analysis of MMJ utilization and its associated outcomes.
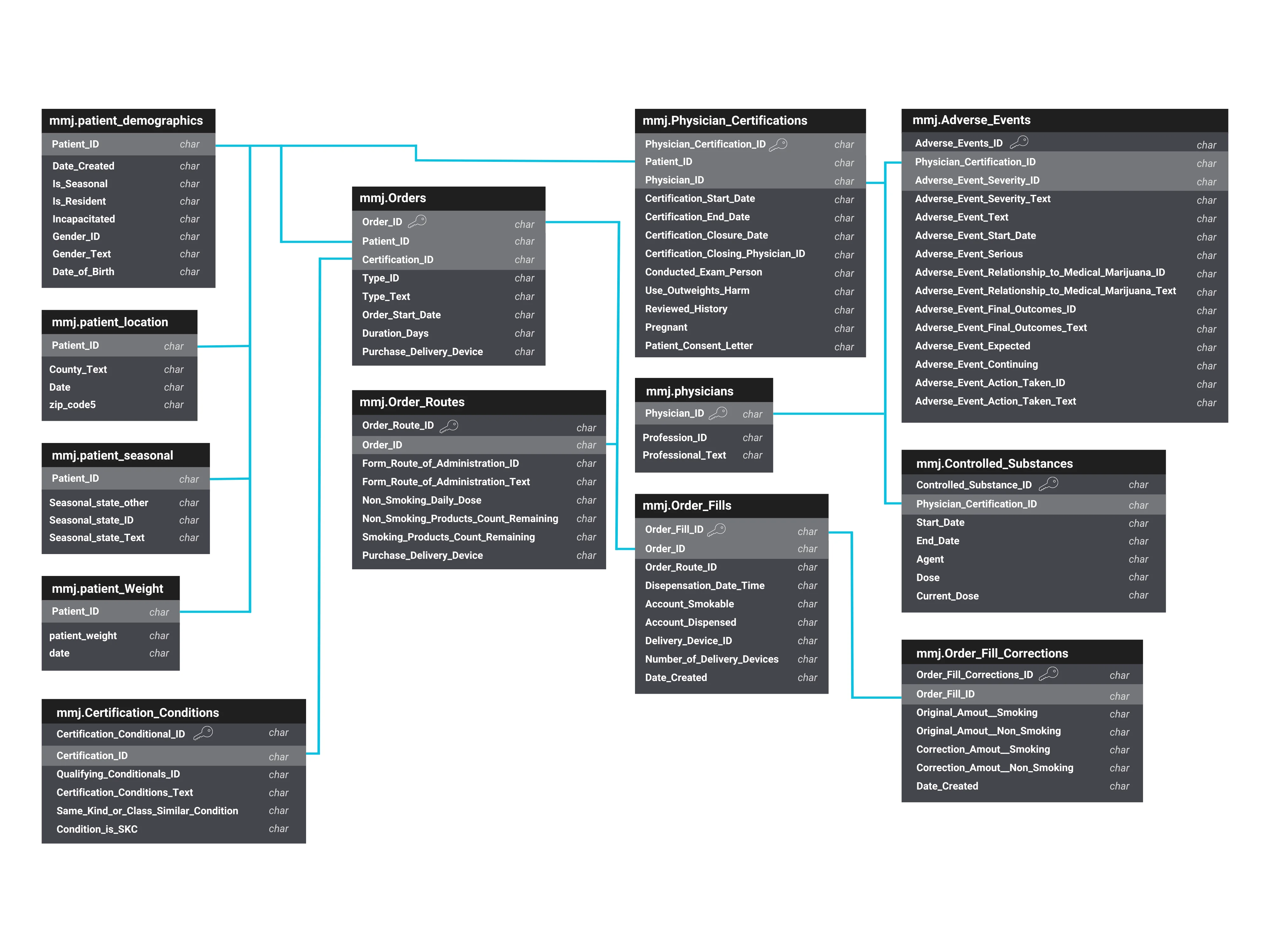
Figure 2. MMUR Data table linkage in MEMORY
MEMORY includes information at the patient-certification level (i.e. each record is a unique combination of an individual patient’s identifier and an individual certification identifier). Figure 3 shows the overall timeline of data ascertainment in building the architecture for patient certification record. While Figure 3 shows 1-year period before Day 0 to measure baseline variables and a 1-year period to ascertain clinical outcomes after the end of the certification period, this 1 year time period is flexible and can be tailored to the specific study design.
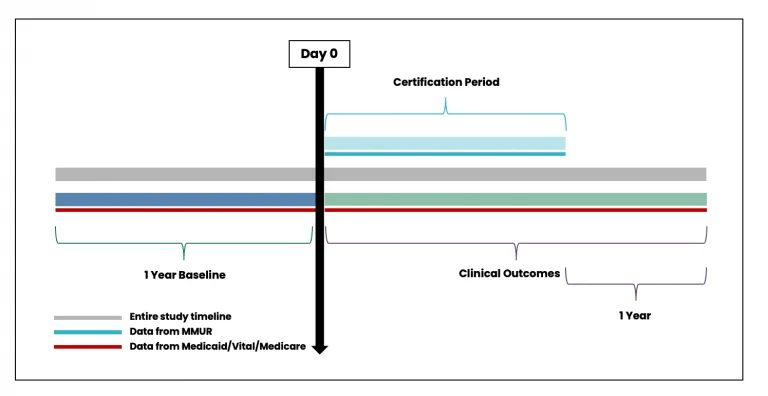
Figure 3. Timeline for data ascertainment from patient certification record
While patient-certifications can occur consecutively if the patient obtains a new certification from their physician (Figure 4), there could also be gaps between the periods of certification (Figure 5). Data from patient-certification record in MEMORY are always anchored on the start date of the patient’s first certification. The look-back and look-forward periods for the ascertainment of baseline variables and clinical outcomes might overlap with periods for another certification record for the same patient.
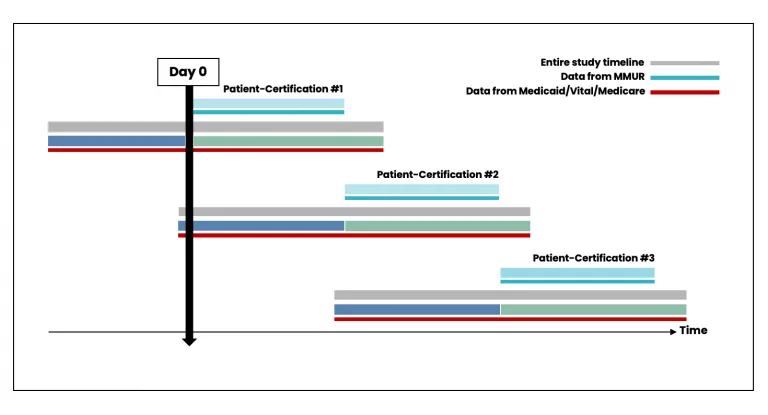
Figure 4. Scenario with no time elapsed between individual certifications
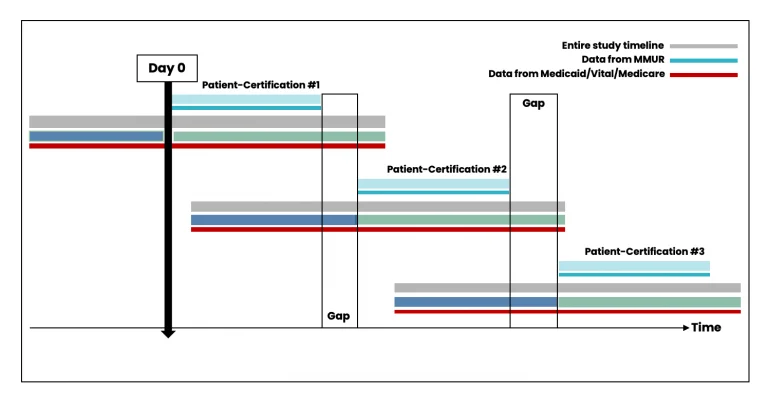
Figure 5. Scenario with time gaps between individual certifications
MEMORY contains information derived from the MMJ prescribing process and from dispensary transactions. Certifications permit patients to legally possess MMJ in the state of Florida and may last up to 210 days. Once a patient is certified, physicians may place orders, analogous to prescriptions, for MMJ, which may last up to 35 or 70 days, depending on the route of administration. Orders may be placed for one or more types of MMJ across as little as one or as many as six unique routes of administration. Figure 6 shows the hierarchical relationship between certifications, orders, and order fills, and depicts how what is prescribed and ultimately dispensed may vary over the duration of the certification.
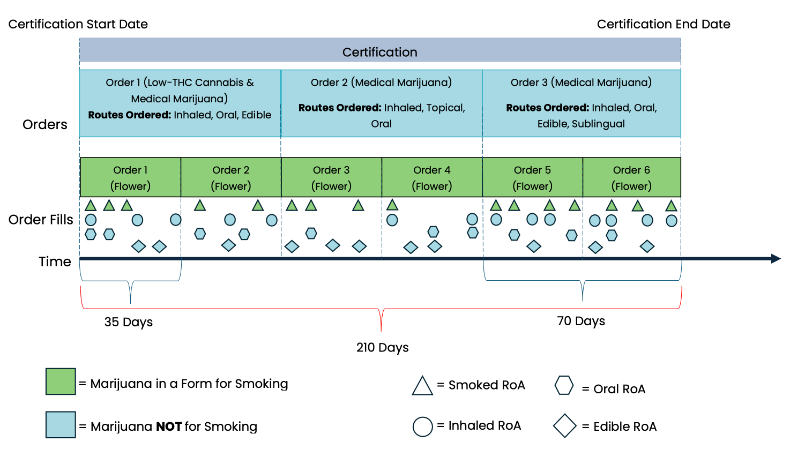
Figure 6. Hierarchical relationship between certifications, orders, and order fills
MEMORY will facilitate the design and execution of myriad study types and designs to answer research questions relating to or based on concurrent prescription medication (from linked prescription claims in AHCA Medicaid and CMS Medicare data) and MMJ utilization (from linked MMUR data). Figure 7 shows the timeline for determining concurrent prescription medication and MMJ utilization.
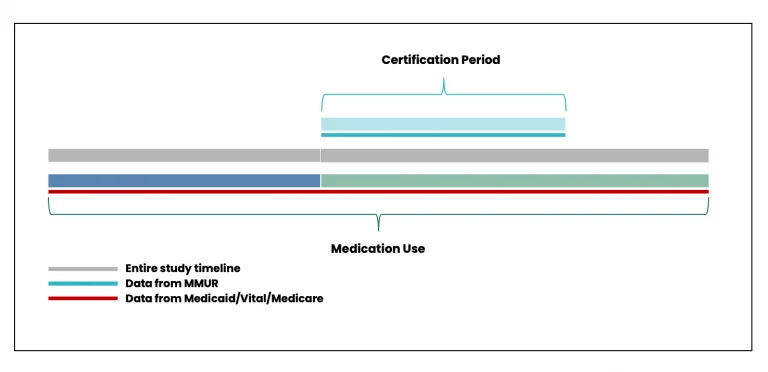
Figure 7. Timeline for determining concurrent prescription medication and MMJ utilization
The MEMORY interlinked data bank will also optimize detail on MMJ use (type, dose, route, from the MMUR) and detail on patient health history, other treatments and outcomes (from linked clinical encounter data such as AHCA Medicaid and CMS Medicare data) and facilitate controlled longitudinal studies on safety and effectiveness outcomes. Importantly, via linkage to other clinical databases including pharmacy dispensing records, researchers will be able to establish control groups to facilitate comparisons of outcomes among patients who have initiated MMJ and patients with similar conditions and health history who are relying on conventional therapeutic approaches alone.
With release of the MMUR data, the Consortium has implemented its long-standing plan to develop MEMORY. This includes MMUR data curation, linkage to other health databases, creation of a data dictionary, and development of analytical cohorts for access by Consortium researchers. As envisioned, MEMORY will then serve to support studies on MMJ effectiveness, safety, utilization, and access. Consortium core faculty plans to have a first set of safety and effectiveness studies completed by the end of 2024.